Apitherapy News - The Internet's Best Source of Information About the Medicinal Use of Bee Products
Tuesday, April 27, 2021
Royal Jelly Protects Brain Against Chronic Stress, Improves Behavioral and Cognitive Impairments
Combined effects of royal jelly and environmental enrichment against stress-induced cognitive and behavioral alterations in male rats: behavioral and molecular studies
Nutr Neurosci. 2021 Apr 5;1-12
Background: Exposure to chronic stress has detrimental effects on cognitive and emotional processing. Also, the neuroprotective influences of environmental enrichment (EE) and royal jelly (RJ) have been indicated in previous studies.
Aims: To our knowledge, to date, there are no studies about the synergistic effects of EE and RJ on cognitive changes induced by stress. Therefore, this study aimed to investigate the protective effects of RJ, and EE on anxiety-like behaviors, cognitive functions, and expression of hippocampal and also prefrontal cortex (PFC) brain-derived neurotrophic factor (BDNF) levels in stressed rats.
Methods: By using restraint and cold temperature, rats were exposed to stressful situations and then subjected to treatment with RJ or/ and EE for 14 days. Stress induction was done 14 days before treatments by placing the rats in the restrainer under 4°C. Following the interventions, anxiety-like behaviors, novel object recognition memory (NORM), inhibitive avoidance performance, hippocampal, and PFC BDNF expression were examined. The plasma corticosterone level of all groups was also evaluated.
Results: Results showed increased plasma corticosterone levels, stress-induced deficits in the NORM and IA tests, and increased anxiety-like behaviors. EE and RJ improved these deficits with a decline in serum corticosterone and also increased BDNF levels in the hippocampus and PFC in stressed ones.
Conclusion: The EE and the RJ prevented the detrimental effects of stress on anxiety-like behaviors and memory processes. These treatments can protect susceptible brain areas against chronic stress via improvement in behavioral and cognitive impairments through mediating BDNF expression.
Monday, April 26, 2021
Wasp and Bee Venom May Help Treat Neurodegenerative Diseases (Alzheimer's Disease and Parkinson's Disease)
Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom
Insects. 2021 Mar 29;12(4):297
The aim of this study was to compare the anti-inflammatory effect of wasp venom (WV) from the yellow-legged hornet (Vespa velutina) with that of bee venom (BV) on BV-2 murine microglial cells.
WV was collected from the venom sac, freeze-dried, and used for in vitro examinations. WV and BV were non-toxic to BV-2 cells at concentrations of 160 and 12 µg/mL or lower, respectively.
Treatment with WV reduced the secretion of nitric oxide and proinflammatory cytokines, including interleukin-6 and tumor necrosis factor alpha, from BV-2 cells activated by lipopolysaccharide (LPS).
Western blot analysis revealed that WV and BV decreased the expression levels of inflammation markers, including inducible nitric oxide synthase and cyclooxygenase-2. In addition, WV decreased the nuclear translocation of nuclear factor κB (NF-κB), which is a key transcription factor in the regulation of cellular inflammatory response.
Cumulatively, the results demonstrated that WV inhibited LPS-induced neuroinflammation in microglial cells by suppressing the NF-κB-mediated signaling pathway, which warrants further studies to confirm its therapeutic potential for neurodegenerative diseases.
Saturday, April 24, 2021
Bee Venom Therapy Used to Treat Lyme Disease
Stinging away Lyme disease with bee venom therapy?
If diagnosed early, antibiotics will wipe out the bacteria left behind in the blood before it spreads through the heart, joints and nervous system.
Now, some patients are turning to bees to take the sting out of this painful and debilitating disease.
One sting -- after another -- after another. These days, Adriana Furey stings herself with ten live honeybees three times a week to get relief from the symptoms of chronic Lyme disease...
Monday, April 19, 2021
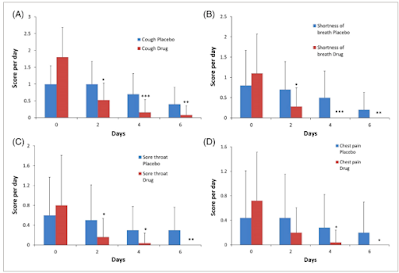
Propolis, Henbane Syrup Helps Treat Symptoms (Cough, Sore Throat, Chest Pain, Shortness of Breath, Fever) of COVID-19 Infection
The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial
Phytother Res. 2021 Apr 15
The outbreak of Coronavirus disease 2019 (COVID-19) has caused a global health crisis. Nevertheless, no antiviral treatment has yet been proven effective for treating COVID-19 and symptomatic supportive cares have been the most common treatment. Therefore, the present study was designed to evaluate the effects of propolis and Hyoscyamus niger L. extract in patients with COVID-19.
This randomized clinical trial was conducted on 50 cases referred to Akhavan and Sepehri Clinics, Kashan university of medical sciences, Iran. Subjects were divided into two groups (intervention and placebo).
This syrup (containing 1.6 mg of methanolic extract along with 450 mg of propolis per 10 mL) was administered three times a day to each patient for 6 days. The clinical symptoms of COVID-19 such as: dry cough, shortness of breath, sore throat, chest pain, fever, dizziness, headache, abdominal pain, and diarrhea were reduced with propolis plus Hyoscyamus niger L. extract than the placebo group. However, the administration of syrup was not effective in the control of nausea and vomiting.
In conclusion, syrup containing propolis and Hyoscyamus niger L. extract had beneficial effects in ameliorating the signs and symptoms of COVID-19 disease, in comparison with placebo groups.
Tuesday, April 06, 2021
Poplar Propolis Extract May Help Treat Obesity
Botanic Origin of Propolis Extract Powder Drives Contrasted Impact on Diabesity in High-Fat-Fed Mice
Antioxidants (Basel). 2021 Mar 9;10(3):411
Propolis extracts are considered as nutraceutical products with potentialities towards obesity and comorbidities management. Nevertheless, propolis extracts composition is highly variable and depends on the botanic origin of plants used by the bees to produce propolis.
This study aims to evaluate the differential effect of poplar propolis extract powder (PPEP), Baccharis propolis extract powder (BPEP), and/ or Dalbergia propolis extract powder (DPEP) on obesity and glucose homeostasis in high-fat-fed mice. PPEP supplementation reduced high-fat (HF)-mediated body weight gain, adiposity index, and improved glucose homeostasis in male C57Bl/6J mice that were submitted to a high-fat diet for 12 weeks, whereas BPEP, DPEP, or a mix of the three PEPs did not modify those parameters.
Adipose tissue (AT) gene expression profiling highlighted an induction of mRNA related to lipid catabolism and an inhibition of mRNA coding for inflammatory markers. Several Nrf2 target genes, coding for antioxidant enzymes, were induced in AT under PPEP effect, but not by other PEP. Interestingly, representative PPEP polyphenols mediated the induction of Nrf2 target genes cell-autonomously in adipocytes, suggesting that this induction may be related to the specific polyphenol content of PPEP.
Whereas PPEP supplementation has demonstrated a clear potential to blunt the onset of obesity and associated comorbidities, other PEPs (from Baccharis and Dalbergia) were inefficient to support their role in preventive nutrition.
Monday, April 05, 2021
Propolis a Safe and Effective Adjunct Treatment for Hospitalized COVID-19 Patients (Coronavirus)
Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial
Biomedicine & Pharmacotherapy, Volume 138, June 2021, 111526
Highlights
• 124 hospitalized COVID-19 patients were randomized into three groups.
• 0, 400 or 800 mg/day of a standardized Brazilian green propolis was provided.
• Adjunct treatment with propolis anticipated hospital release by five to six days.
• The 800 mg propolis dose reduced kidney damage associated with COVID-19.
• Propolis was safe and effective as an adjunct treatment.
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) promotes challenging immune and inflammatory phenomena. Though various therapeutic possibilities have been tested against coronavirus disease 2019 (COVID-19), the most adequate treatment has not yet been established. Propolis is a natural product with considerable evidence of immunoregulatory and anti-inflammatory activities, and experimental data point to potential against viral targets. We hypothesized that propolis can reduce the negative effects of COVID-19.
Methods
In a randomized, controlled, open-label, single-center trial, hospitalized adult COVID-19 patients were treated with a standardized green propolis extract (EPP-AF®️) as an adjunct therapy. Patients were allocated to receive standard care plus an oral dose of 400 mg or 800 mg/day of green propolis for seven days, or standard care alone. Standard care included all necessary interventions, as determined by the attending physician. The primary end point was the time to clinical improvement, defined as the length of hospital stay or oxygen therapy dependency duration. Secondary outcomes included acute kidney injury and need for intensive care or vasoactive drugs. Patients were followed for 28 days after admission.
Results
We enrolled 124 patients; 40 were assigned to EPP-AF®️ 400 mg/day, 42 to EPP-AF®️ 800 mg/day, and 42 to the control group. The length of hospital stay post-intervention was shorter in both propolis groups than in the control group; lower dose, median 7 days versus 12 days (95% confidence interval [CI] −6.23 to −0.07; p = 0.049) and higher dose, median 6 days versus 12 days (95% CI −7.00 to −1.09; p = 0.009). Propolis did not significantly affect the need for oxygen supplementation. In the high dose propolis group, there was a lower rate of acute kidney injury than in the controls (4.8 vs 23.8%), (odds ratio [OR] 0.18; 95% CI 0.03–0.84; p = 0.048). No patient had propolis treatment discontinued due to adverse events.
Conclusions
Addition of propolis to the standard care procedures resulted in clinical benefits for the hospitalized COVID-19 patients, especially evidenced by a reduction in the length of hospital stay. Consequently, we conclude that propolis can reduce the impact of COVID-19.
• 124 hospitalized COVID-19 patients were randomized into three groups.
• 0, 400 or 800 mg/day of a standardized Brazilian green propolis was provided.
• Adjunct treatment with propolis anticipated hospital release by five to six days.
• The 800 mg propolis dose reduced kidney damage associated with COVID-19.
• Propolis was safe and effective as an adjunct treatment.
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) promotes challenging immune and inflammatory phenomena. Though various therapeutic possibilities have been tested against coronavirus disease 2019 (COVID-19), the most adequate treatment has not yet been established. Propolis is a natural product with considerable evidence of immunoregulatory and anti-inflammatory activities, and experimental data point to potential against viral targets. We hypothesized that propolis can reduce the negative effects of COVID-19.
Methods
In a randomized, controlled, open-label, single-center trial, hospitalized adult COVID-19 patients were treated with a standardized green propolis extract (EPP-AF®️) as an adjunct therapy. Patients were allocated to receive standard care plus an oral dose of 400 mg or 800 mg/day of green propolis for seven days, or standard care alone. Standard care included all necessary interventions, as determined by the attending physician. The primary end point was the time to clinical improvement, defined as the length of hospital stay or oxygen therapy dependency duration. Secondary outcomes included acute kidney injury and need for intensive care or vasoactive drugs. Patients were followed for 28 days after admission.
Results
We enrolled 124 patients; 40 were assigned to EPP-AF®️ 400 mg/day, 42 to EPP-AF®️ 800 mg/day, and 42 to the control group. The length of hospital stay post-intervention was shorter in both propolis groups than in the control group; lower dose, median 7 days versus 12 days (95% confidence interval [CI] −6.23 to −0.07; p = 0.049) and higher dose, median 6 days versus 12 days (95% CI −7.00 to −1.09; p = 0.009). Propolis did not significantly affect the need for oxygen supplementation. In the high dose propolis group, there was a lower rate of acute kidney injury than in the controls (4.8 vs 23.8%), (odds ratio [OR] 0.18; 95% CI 0.03–0.84; p = 0.048). No patient had propolis treatment discontinued due to adverse events.
Conclusions
Addition of propolis to the standard care procedures resulted in clinical benefits for the hospitalized COVID-19 patients, especially evidenced by a reduction in the length of hospital stay. Consequently, we conclude that propolis can reduce the impact of COVID-19.