Thursday, November 28, 2019
Indonesian Stingless Bee Propolis is a Potential Non-Steroidal Anti-Inflammatory Drug
Anti-inflammatory activity of Tetragronula species from Indonesia
Saudi J Biol Sci. 2019 Nov;26(7):1531-153
Anti-inflammatory drugs inhibit inflammation, particularly those classified as nonsteroidal anti-inflammatory drugs (NSAIDs). Several studies have reported that propolis has both anti-ulcerogenic and anti-inflammatory effects.
In this study, we investigated the bioactive compound and in vivo anti-inflammatory properties of both smooth and rough propolis from Tetragronula sp. To further identify anti-inflammatory markers in propolis, LC-MS/MS was used, and results were analyzed by Mass Lynx 4.1. Rough and smooth propolis of Tetragonula sp. were microcapsulated with maltodextrin and arabic gum. Propolis microcapsules at dose 25-200 mg/kg were applied for carrageenan-induced rat's paw-inflammation model.
Data were analyzed by one-way ANOVA and Kruskal-Wallis statistical tests. LC-MS/MS experiments identified seven anti-inflammatory compounds, including [6]-dehydrogingerdione, alpha-tocopherol succinate, adhyperforin, 6-epiangustifolin, deoxypodophyllotoxin, kurarinone, and xanthoxyletin. Our results indicated that smooth propolis at 50 mg/kg inhibited inflammation to the greatest extent, followed by rough propolis at a dose of 25 mg/kg. SPM and RPM with the dose of 25 mg/kg had inflammatory inhibition value of 62.24% and 58.12%, respectively, which is comparable with the value 70.26% of sodium diclofenac with the dose of 135 mg/kg.
This study suggests that propolis has the potential candidate to develop as a non-steroid anti-inflammatory drug.
Friday, November 22, 2019
What is Apitherapy?
New Bee-Centric Spa Has Us Buzzing
Worker B Wellness offers holistic services using bee-derived products and provides free educational sessions about bees
Apitherapy is a form of alternative medicine that uses honey bee products such as honey, propolis, pollen, royal jelly, and bee venom. This type of therapy has existed for thousands of years. According to The American Apitherapy Society Inc., rock art created in ancient times illustrated that early hunter-gatherers viewed honeybees as a source of natural medicine. In fact, apitherapy was used to treat arthritis and other joint problems, which is still practiced today.
Romanelli has always had an interest in bees. She laughs as she says her family was “urban hippies”—always growing fresh produce and eating organic, local foods.
“My interest in bees is really an interest in the product they provide and also how they work in community with each other, which is really fascinating,” Romanelli says. “Each bee has a specific job in the hive to help the whole as a superorganism. So, when we were looking at this, we’re like, ‘OK, so Worker B Wellness is a superorganism—we’re all doing something to help the concept of the hive.”...
Thursday, November 21, 2019
Can Bee Stings Treat Lyme Disease?
Treatments for chronic Lyme disease are controversial and expensive. As a last resort, some patients are pursuing this unproven and painful alternative.
TEXAS MONTHLY, DECEMBER 2019
Avery was 41 and had been diagnosed with Lyme in 2013, though her symptoms dated back years before that. She told me that it had taken a few months to work up to ten stings; she thought the bee venom was setting off something called a Jarisch-Herxheimer reaction, a response to antimicrobials like antibiotics, in which the bacteria being destroyed release their contents into the bloodstream and cause flu-like symptoms. (It’s not scientifically documented whether venom in fact triggers the same reaction.) “I’m starting to handle it better than at first,” Avery said. “Like I’m not—knock on wood—getting as sick now because the bacterial load is going down, and I think I have a pretty good detox routine.” On the day following a stinging session, the women typically soak in Epsom salt baths, dry brush the skin around their lymph glands, and take coffee enemas—an effort they believe will expel toxins.
Like a nurse giving a vaccine, Gschwind held a bee in tweezers as Avery removed her ice pack. “Okay, ready?” Gschwind asked. “One, two, three.” She held the bee’s butt about an inch to the left of Avery’s lower spine and waited for the tiny stinger to pierce the skin. Then she pulled away the bee, now missing its stinger, which was still drilling into Avery’s back. The vibrating fuzzball was doomed without its barb, and Gschwind placed it in some soapy water to hasten its inevitable end. Afterward, Avery grimaced and breathed deeply as she returned the ice pack to her lower spine, preparing for the next sting.
This process would continue until the bottom portion of Avery’s spine looked like a clear highway running between two parallel rows of five inflamed bumps. Each protrusion was dotted with a stinger, moving like a pumpjack atop a tiny, rising hill, as it continued to gently, rhythmically, excrete venom. Twenty minutes later, when the barbs had done their job and stopped moving, Gschwind extracted them. Then Avery went through the same procedure to sting Gschwind.
While everything about this routine was taxing—the stinging, the seeming Herxheimer reaction, collecting bees and keeping them in her apartment—Gschwind didn’t see much of an alternative. She could not sit on the couch and groan in pain year in and year out while doctors told her she was fine, or that she should be fine, or that she would feel better in time. She’d tried that already. “What am I going to do? Because this isn’t fair. I deserve to have a life, to be functional,” she said. “Well, I guess I’m going to stick myself with bees.”...
Labels:
Bee Venom
Monday, November 18, 2019
Black Seed Oil, Honey, Whey Protein Increase Antioxidant Activity
Effect of black seed oil, honey, whey protein concentrate and their interaction on antioxidant activity, elastic modulus and creaming index of O/W emulsions
Journal of Dispersion Science and Technology
An emulsion based on a combination of highly-valued black seed oil (BSO) and honey shall be introduced as an emerging nutraceutical since the emulsion could be an efficient carrier for bioactive compounds from both.
This study aimed to determine effect of BSO, honey, whey protein concentrate (WPC) and their interaction on antioxidant activity (i.e. IC50, the concentration of sample required to scavenge 50% of 2,2-diphenyl-1-picrylhydrazyl free radicals), elastic modulus (G′) and creaming index (CI) of O/W emulsions by means of a response surface methodology. Twenty emulsions were ultrasonically prepared by using various combinations of BSO (10–20%), honey (10–20% and WPC (2–6%) based on a central composite design. Regression analysis (R2 = 0.92–1.00) revealed that a decrease in the IC50 was mainly due to significant (p < 0.05) linear effect of honey and WPC.
The quadratic effect of WPC significantly increased the G′ yet decreased the CI. Synergistic effects of BSO-honey on IC50 and G′ were also significant (p < 0.05). However, the antagonistic effect (p < 0.05) of honey-WPC seemed to increase the IC50. By using the fitted quadratic models, the optimized levels of BSO (20.0%), honey (18.2%) and WPC (6.0%) were proposed and predicted to provide the desired emulsion with IC50 = 0.12 mg/ml, G′ = 606.65 Pa and CI = 1.45%. These values were successfully validated with their respective experimental values.
Labels:
Honey
Monday, November 11, 2019
Propolis an Eco-Friendly Antibacterial Coating for Wound Sutures
Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco-friendly solution with wound healing potential against surgical site infections (SSIs)
Turk J Med Sci. 2019 Oct 27
BACKGROUND/AIM:
Bacterial adherence to a suture material is one of the main reasons that cause surgical site infections. An antibacterial suture material with enhanced wound healing function may prevent the surgical site from infections. Thus, the present study was aimed to investigate the synergistic effect of propolis and biogenic metallic nanoparticles when combined with silk sutures for biomedical use.
MATERIALS AND METHODS:
Silver nanoparticle (AgNP) synthesis was carried out via a microbial-mediated biological route and impregnated on propolis-loaded silk sutures using an in situ process. silk sutures fabricated with propolis and biosynthesized AgNPs (bioAgNP-propolis coated sutures) were intensively characterized using Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDS), Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). Antibacterial characteristics of the bioAgNP-propolis coated sutures was evaluated using agar plate method. Biocompatibility of the bioAgNP-propolis coated sutures was evaluated using 3T3 fibroblast cells and their wound healing potential was also investigated.
RESULTS:
BioAgNP-propolis coated sutures displayed potent antibacterial activity against pathogenic Gram negative and Gram positive bacteria; Escherichia coli and Staphylococcus aureus, respectively. BioAgNP-propolis coated silk sutures were found to be biocompatible with 3T3 fibroblast cell culture. In vitro wound healing scratch assay was also demonstrated that the extract of bioAgNP-propolis coated sutures stimulated the 3T3 fibroblasts? cell proliferation.
CONCLUSION:
Coating the silk sutures with propolis and biogenic AgNPs gained an effective antibacterial capacity to surgical sutures besides providing biocompatibility and wound healing activity.
Turk J Med Sci. 2019 Oct 27
BACKGROUND/AIM:
Bacterial adherence to a suture material is one of the main reasons that cause surgical site infections. An antibacterial suture material with enhanced wound healing function may prevent the surgical site from infections. Thus, the present study was aimed to investigate the synergistic effect of propolis and biogenic metallic nanoparticles when combined with silk sutures for biomedical use.
MATERIALS AND METHODS:
Silver nanoparticle (AgNP) synthesis was carried out via a microbial-mediated biological route and impregnated on propolis-loaded silk sutures using an in situ process. silk sutures fabricated with propolis and biosynthesized AgNPs (bioAgNP-propolis coated sutures) were intensively characterized using Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDS), Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). Antibacterial characteristics of the bioAgNP-propolis coated sutures was evaluated using agar plate method. Biocompatibility of the bioAgNP-propolis coated sutures was evaluated using 3T3 fibroblast cells and their wound healing potential was also investigated.
RESULTS:
BioAgNP-propolis coated sutures displayed potent antibacterial activity against pathogenic Gram negative and Gram positive bacteria; Escherichia coli and Staphylococcus aureus, respectively. BioAgNP-propolis coated silk sutures were found to be biocompatible with 3T3 fibroblast cell culture. In vitro wound healing scratch assay was also demonstrated that the extract of bioAgNP-propolis coated sutures stimulated the 3T3 fibroblasts? cell proliferation.
CONCLUSION:
Coating the silk sutures with propolis and biogenic AgNPs gained an effective antibacterial capacity to surgical sutures besides providing biocompatibility and wound healing activity.
Labels:
Propolis
Friday, November 08, 2019
Honey and Its Combination with Metformin Prevents Hyperglycemia, Stimulates Insulin Secretion, Reduces Liver Fat Accumulation, Attenuates Liver Injury and Kidney Damage
Combination of honey with metformin enhances glucose metabolism and ameliorates hepatic and nephritic dysfunction in STZ-induced diabetic mice
Food Funct. 2019 Nov 5
Honey is a natural sweetener that contains a large amount of monosaccharides such as glucose and fructose, as well as small amounts of disaccharides and trisaccharides such as sucrose and pine trisaccharides. In addition to carbohydrates, honey also contains vitamins, minerals, enzymes, amino acids, and polyphenols including phenolic acids and flavonoids.
The polyphenols in honey have been proved to have great antioxidant activity, besides inhibiting α-glycosidase activity and improving blood-lipid metabolism. However, whether it is safe for diabetic patients to consume honey remains controversial.
This study investigated the effects of honey, metformin and their combination on the characteristic pathological changes and glucose metabolism in STZ-induced diabetic mice over five weeks.
Our results showed that honey and its combination with metformin could prevent hyperglycemia, stimulate insulin secretion, reduce liver fat accumulation, attenuate liver injury and kidney damage in STZ-induced diabetic mice.
Moreover, treatment with honey or combination of honey and metformin significantly enhanced glucokinase (GK) activity (p < 0.05), and meanwhile suppressed the activities of glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK), pyruvate carboxylase (PC) and pyruvate dehydrogenase kinases (PDK) (p < 0.05) in diabetic mice.
Thursday, November 07, 2019
Bee Venom Has Anticoagulant Properties
The anticoagulant effect of Apis mellifera phospholipase A2 is inhibited by CORM-2 via a carbon monoxide-independent mechanism
J Thromb Thrombolysis. 2019 Nov 2
Bee venom phospholipase A2 (PLA2) has potential for significant morbidity. Ruthenium (Ru)-based carbon monoxide releasing molecules (CORM) inhibit snake venoms that are anticoagulant and contain PLA2. In addition to modulating heme-bearing proteins with carbon monoxide, these CORM generate reactive Ru species that form adducts with histamine residues resulting in changes in protein function.
This study sought to identify anticoagulant properties of bee venom PLA2 via catalysis of plasma phospholipids required for thrombin generation. Another goal was to determine if Ru-based CORM inhibit bee venom PLA2 via carbon monoxide release or via potential binding of reactive Ru species to a key histidine residue in the catalytic site of the enzyme. Anticoagulant activity of bee venom PLA2 was assessed via thrombelastography with normal plasma.
Bee venom PLA2 was then exposed to different CORM and a metheme forming agent and anticoagulant activity was reassessed. Using Ru, boron and manganese-based CORM and a metheme forming agent, it was demonstrated that it was unlikely that carbon monoxide interaction with a heme group attached to PLA2 was responsible for inhibition of anticoagulant activity by Ru-based CORM. Exposure of PLA2 to a Ru-based CORM in the presence of histidine-rich human albumin resulted in loss of inhibition of PLA2.
Ru-based CORM likely inhibit bee venom PLA2 anticoagulant activity via formation of reactive Ru species that bind to histidine residues of the enzyme.
J Thromb Thrombolysis. 2019 Nov 2
Bee venom phospholipase A2 (PLA2) has potential for significant morbidity. Ruthenium (Ru)-based carbon monoxide releasing molecules (CORM) inhibit snake venoms that are anticoagulant and contain PLA2. In addition to modulating heme-bearing proteins with carbon monoxide, these CORM generate reactive Ru species that form adducts with histamine residues resulting in changes in protein function.
This study sought to identify anticoagulant properties of bee venom PLA2 via catalysis of plasma phospholipids required for thrombin generation. Another goal was to determine if Ru-based CORM inhibit bee venom PLA2 via carbon monoxide release or via potential binding of reactive Ru species to a key histidine residue in the catalytic site of the enzyme. Anticoagulant activity of bee venom PLA2 was assessed via thrombelastography with normal plasma.
Bee venom PLA2 was then exposed to different CORM and a metheme forming agent and anticoagulant activity was reassessed. Using Ru, boron and manganese-based CORM and a metheme forming agent, it was demonstrated that it was unlikely that carbon monoxide interaction with a heme group attached to PLA2 was responsible for inhibition of anticoagulant activity by Ru-based CORM. Exposure of PLA2 to a Ru-based CORM in the presence of histidine-rich human albumin resulted in loss of inhibition of PLA2.
Ru-based CORM likely inhibit bee venom PLA2 anticoagulant activity via formation of reactive Ru species that bind to histidine residues of the enzyme.
Labels:
Bee Venom
Tuesday, November 05, 2019
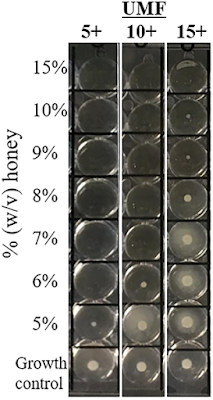
UMF Value a Not Reliable Indicator of Manuka Honey Antibacterial Activity
Antibacterial activity of varying UMF-graded Manuka honeys
PLoS One. 2019 Oct 25;14(10):e0224495
Honey has been used as a traditional remedy for skin and soft tissue infections due to its ability to promote wound healing. Manuka honey is recognized for its unusually abundant content of the antibacterial compound, methylglyoxal (MGO).
The Unique Manuka Factor (UMF) grading system reflects the MGO concentration in Manuka honey sold commercially. Our objective was to observe if UMF values correlated with the antibacterial activity of Manuka honey against a variety of pathogens purchased over the counter.
The antibacterial effect of Manuka honey with UMF values of 5+, 10+, and 15+ from the same manufacturer was assessed by the broth microdilution method. Minimum inhibitory concentration (MIC) values were determined against 128 isolates from wound cultures representing gram-positive, gram-negative, drug-susceptible, and multi-drug resistant (MDR) organisms.
Lower MICs were observed with UMF 5+ honey for staphylococci (n = 73, including 25 methicillin-resistant S. aureus) and Pseudomonas aeruginosa (n = 22, including 10 MDR) compared to UMF 10+ honey (p < 0.05) and with UMF 10+ compared to UMF 15+ (p = 0.01). For Enterobacteriaceae (n = 33, including 14 MDR), MIC values were significantly lower for UMF 5+ or UMF 10+ compared to UMF 15+ honey (p < 0.01). MIC50 for UMF 5+, UMF 10+, and UMF 15+ honey against staphylococci was 6%, 7%, and 15%, and for Enterobacteriaceae was 21%, 21%, and 27%, respectively.
For Pseudomonas aeruginosa MIC50 was 21% and MIC90 was 21-27% for all UMFs. Manuka honey exhibited antimicrobial activity against a spectrum of organisms including those with multi-drug resistance, with more potent activity overall against gram-positive than gram-negative bacteria.
Manuka honey with lower UMF values, in our limited sampling, paradoxically demonstrated increased antimicrobial activity among the limited samples tested, presumably due to changes in MGO content of honey over time. The UMF value by itself may not be a reliable indicator of antibacterial effect.
PLoS One. 2019 Oct 25;14(10):e0224495
Honey has been used as a traditional remedy for skin and soft tissue infections due to its ability to promote wound healing. Manuka honey is recognized for its unusually abundant content of the antibacterial compound, methylglyoxal (MGO).
The Unique Manuka Factor (UMF) grading system reflects the MGO concentration in Manuka honey sold commercially. Our objective was to observe if UMF values correlated with the antibacterial activity of Manuka honey against a variety of pathogens purchased over the counter.
The antibacterial effect of Manuka honey with UMF values of 5+, 10+, and 15+ from the same manufacturer was assessed by the broth microdilution method. Minimum inhibitory concentration (MIC) values were determined against 128 isolates from wound cultures representing gram-positive, gram-negative, drug-susceptible, and multi-drug resistant (MDR) organisms.
Lower MICs were observed with UMF 5+ honey for staphylococci (n = 73, including 25 methicillin-resistant S. aureus) and Pseudomonas aeruginosa (n = 22, including 10 MDR) compared to UMF 10+ honey (p < 0.05) and with UMF 10+ compared to UMF 15+ (p = 0.01). For Enterobacteriaceae (n = 33, including 14 MDR), MIC values were significantly lower for UMF 5+ or UMF 10+ compared to UMF 15+ honey (p < 0.01). MIC50 for UMF 5+, UMF 10+, and UMF 15+ honey against staphylococci was 6%, 7%, and 15%, and for Enterobacteriaceae was 21%, 21%, and 27%, respectively.
For Pseudomonas aeruginosa MIC50 was 21% and MIC90 was 21-27% for all UMFs. Manuka honey exhibited antimicrobial activity against a spectrum of organisms including those with multi-drug resistance, with more potent activity overall against gram-positive than gram-negative bacteria.
Manuka honey with lower UMF values, in our limited sampling, paradoxically demonstrated increased antimicrobial activity among the limited samples tested, presumably due to changes in MGO content of honey over time. The UMF value by itself may not be a reliable indicator of antibacterial effect.
Labels:
Honey
Subscribe to:
Posts (Atom)